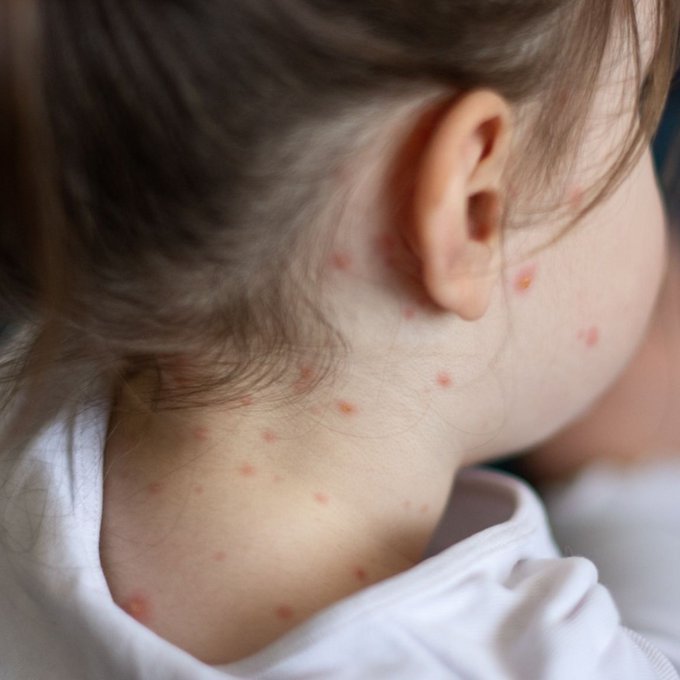
Brief by Shorts91 Newsdesk / 06:19pm on 26 Feb 2026,Thursday Health & Wellness
A leaked internal report from the UK Health Security Agency has revealed 340 suspected measles cases in London since the start of 2026, with 123 confirmed. The outbreak is particularly alarming as 34 confirmed cases were directly linked to a single school in Enfield, north London, between January 20 and February 7. The majority of cases affect children aged one to four, with 42 cases recorded among children from the most deprived areas. Between January 2024 and February 2026, England recorded 1,117 measles cases overall, with 597 in London alone, causing the UK to lose its measles elimination status. Health officials are urgently urging parents to vaccinate children, citing dangerously low MMRV vaccine uptake in areas including Hackney and Enfield. (PC: X)
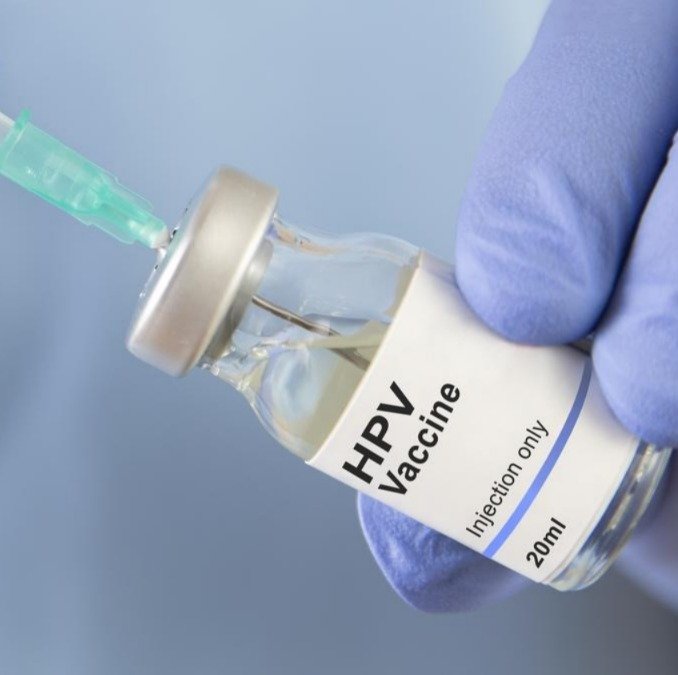
Brief by Shorts91 Newsdesk / 02:49am on 25 Feb 2026,Wednesday Health & Wellness
India's Union Health Ministry is set to launch a nationwide, free Human Papillomavirus (HPV) vaccination programme targeting adolescent girls aged 14 years. The vaccination will be entirely voluntary and free of cost, ensuring equitable access across all socio-economic groups. India will deploy Gardasil, a quadrivalent HPV vaccine offering protection against HPV types 16 and 18, the primary causes of cervical cancer as well as types 6 and 11. Health Ministry officials confirmed that global and Indian scientific evidence supports a single-dose regimen as providing robust and durable protection when administered to girls within the recommended age group, marking a landmark step in India's public health and women's healthcare landscape. (PC: Getty Images)

Brief by Shorts91 Newsdesk / 07:32am on 06 Feb 2026,Friday Health & Wellness
H5N1 avian influenza has been detected in Chennai following the sudden death of hundreds of crows across the city. Officials tested bird samples from Adyar and confirmed Highly Pathogenic Avian Influenza. The state issued a public advisory and began containment steps. Residents have been told not to touch dead birds and to inform authorities about new cases. Officials ordered safe disposal of carcasses through burning or deep burial. The Union Animal Husbandry Ministry asked Tamil Nadu to apply a “One-Health approach” linking animal, human, and wildlife departments. The ministry said the situation “demands urgent attention” and called for wider field surveillance. (PC: India Today)

Brief by Shorts91 Newsdesk / 06:27am on 30 Jan 2026,Friday Health & Wellness
The World Health Organization said there is no need for travel or trade restrictions after India reported two Nipah virus cases. The agency said the outbreak poses a low risk. “There is no evidence of increased human-to-human transmission,” WHO said. The cases were reported from North 24 Parganas in West Bengal. It added that the patients did not travel while showing symptoms. Some Asian countries have increased airport screening. Nipah mainly spreads from bats through contaminated food or close contact. It can cause fever, brain swelling, and breathing problems. There is no approved vaccine yet, but research is ongoing. (PC: Reuters)

Brief by Shorts91 Newsdesk / 10:30am on 29 Jan 2026,Thursday Health & Wellness
Indonesia has begun using thermal scanners at major airports after two Nipah virus cases were confirmed in India, officials said. The measures include checks at Bali’s international airport, which handles large numbers of foreign visitors. Last year, about 5.89 million overseas tourists visited Bali, including many from India. Tourists told Reuters the move reflects lessons learned from the COVID-19 pandemic, though some said the risk remains low. Nipah is carried by fruit bats and animals such as pigs. It can cause fever and brain inflammation. The virus can spread between people, but usually requires close and prolonged contact. (PC: Reuters)
Brief by Shorts91 Newsdesk / 03:39am on 29 Jan 2026,Thursday Health & Wellness
UK health authorities have issued a health warning about the Nipah virus after two cases were confirmed in West Bengal, India, prompting increased airport screening and surveillance across several Asian countries. The UK Health Security Agency (UKHSA) says the risk to the UK public remains very low, as no cases have been detected there, but emphasises travellers should be aware. Nipah is a zoonotic virus transmitted from animals like fruit bats to humans, through contaminated food, or via close contact, and has a high fatality rate (40–75 %). Initial symptoms include fever, headache, and respiratory issues, which can worsen to encephalitis or brain inflammation. There is no specific treatment or vaccine currently available, making vigilance essential.

Brief by Shorts91 Newsdesk / 06:41pm on 28 Jan 2026,Wednesday Health & Wellness
Indian officials announced they have contained a Nipah virus outbreak after confirming two cases in West Bengal state, saying enhanced surveillance, lab testing and tracing of 196 contacts — all testing negative — helped control spread. Nipah is a highly fatal virus with a 40–75% death rate and no vaccine, transmitted from animals like fruit bats and pigs to humans. The health ministry said the situation is under constant monitoring with measures in place. Several Asian countries have stepped up airport health screenings as a precaution. (PC: Reuters)

Brief by Shorts91 Newsdesk / 04:15pm on 23 Jan 2026,Friday Health & Wellness
West Bengal faces a Nipah virus outbreak with five confirmed cases in Barasat near Kolkata, prompting authorities to place 100 individuals under home quarantine. The infected include two nurses in ICU, a doctor, another nurse, and a healthcare worker, now receiving specialized care at Beleghata infectious diseases hospital. The Union Health Ministry deployed a National Joint Outbreak Response Team and shared containment protocols. Key precautions include thoroughly washing fruits, avoiding raw date palm sap, maintaining hand hygiene, and not consuming ground fruits showing bite marks. Symptoms appear within 4-14 days, resembling flu initially before potentially causing serious neurological complications. With no approved vaccine or cure, rapid detection and strict isolation remain critical for containment. (PC:X)
.jpeg)
Brief by Shorts91 Newsdesk / 04:34am on 23 Jan 2026,Friday Health & Wellness
At least 22 people in Indore’s Mhow area fell ill after consuming contaminated drinking water, prompting hospitalisation and medical monitoring. Nine were admitted, while others receive treatment at home as authorities survey affected neighbourhoods. This new wave comes weeks after a larger waterborne disease crisis linked to contaminated pipelines in Bhagirathpura that earlier killed at least 15 people and hospitalised many more amid diarrhoea and vomiting outbreaks. The local administration, led by District Collector Shivam Verma, deployed health teams and began a rapid response to contain further spread. A high-level probe into the causes and accountability is underway. (PC: PTI)
Brief by Shorts91 Newsdesk / 02:24pm on 12 Jan 2026,Monday Health & Wellness
Two suspected Nipah virus cases have been detected at Barasat Hospital in West Bengal, officials said on Monday. State health teams visited the hospital to assess the situation. The Union health secretary reviewed the matter with senior state officers. A national outbreak response team has been sent to support containment steps. The team includes experts from NIV Pune, NIE Chennai, AIIMS Kalyani, and institutes in Kolkata. The NCDC has activated its Public Health Emergency Operations Centre to coordinate action. The Centre has shared guidelines with the state disease unit. Union Health Minister J P Nadda wrote to Chief Minister Mamata Banerjee and assured full support, including lab help, surveillance, and contact tracing. (PC: AI-Generated)